First, what’s corrosion and why is it important to prevent it? Corrosion is the deterioration of a metal due to it rusting. This can happen when metals come in contact with water, especially salty water, as in oceans and seas. This is where anodes for aluminum boatss are far superior in the prevention of this problem. Using them makes it possible for your equipment to grow longer. That means it is designed to function for many years to come, potentially saving you time and money in repairs.
So, now let’s understand how aluminium alloy anodes actually prevent corrosion. To do this, we need to learn more about what causes corrosion, to begin with. Corrosion is common when two different metals make contact in a salty environment, such as seawater. When they touch, one metal may corrode faster than the other. This type of rusting is known as galvanic corrosion.
The moment you connect a sacrificial anode made out of an aluminum alloy to your metallic equipment, it becomes its cover. It protects the metal from rusting. The great thing is that the anode will corrode instead of the metal it’s protecting. That is, the anode literally gives itself up to protect the precious metal. As long as the aluminum zinc anode remains attached to the metal, the metal will be protected against corrosion.
As we said, another reason we switch to aluminum alloy anodes is their life cycle. They are built to last, particularly in marine environments. Equipment in the water exposes it to myriad hazards, from saltwater to bright sunlight to extreme temperature. Any of these things can wear on equipment over time.
Aluminum alloy anodes are robust due to their unique material composition consisting of aluminum, zinc, and other elements. This needs to be a hard and durable substance. In addition, aluminum alloy anodes are meant to be replaced on a regular basis. It means that you will ensure protection for your from corrosion and you will not have to worry about it anymore.
If metals need protection against rust, you have many options. Aluminum alloy anodes have generally been the best option for multiple key reasons. First, they are incredibly effective at preventing corrosion, which we’ve already mentioned. Such effectiveness is essential in retaining your equipment well.
Aluminum alloy anodes not only have protective properties but are also cost-effective. They won't break the bank at all and are easily replaceable whenever necessary. Opting for aluminum alloy anodes could be the most economical choice in the changing over process — it's going to prevent a lot of cash on remodels and substitutions in the future.
SME offers an extensive range of Plate Heat Exchanger (aluminum alloy anode) maintenance services tailored to ensure the durability and effectiveness of your equipment. The services we offer include expert cleaning, meticulous inspections, precise pressure testing, and the most advanced cleaning In Place (CIP) processes that permit efficient in-line cleaning, without the requirement to dismantle the equipment.With a 5,000-square-meter workshop and a massive $10 million inventory, we are well-equipped to handle projects of all sizes. Our huge capacity, coupled with our long-standing experience and the most up-to-date service technology, allows us to provide a 12-month warranty for every PHE projects.We're dedicated to quality and precision. This allows us to minimize risks and increase the lifespan of the heat exchangers you have installed.
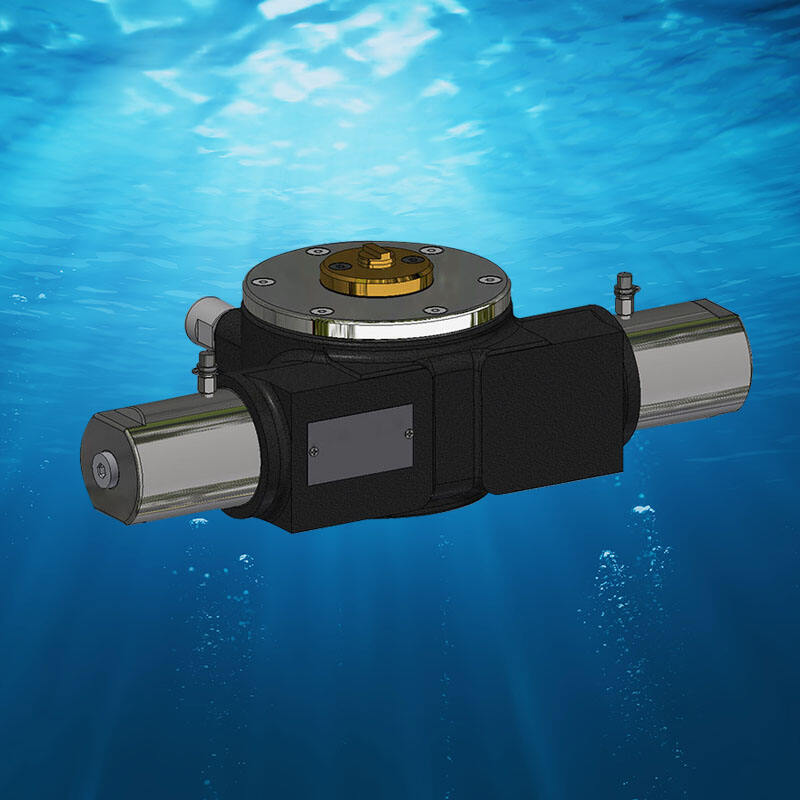
SME's ICCP (Impressed Current Cathodic Protection) Systems provide solid corrosion protection due to the latest technology and many years of expertise in the field. As a leading manufacturer in China we guarantee high quality and aluminum alloy anode through the accumulation of expertise gained by handling hundreds of vessel projects every year.We offer comprehensive ICCP solutions, including all anodes, spare parts and electrodes for reference. Furthermore, SME provides expert modification repairs, maintenance, and modification services to ensure your system runs at peak performance throughout its lifetime. SME also provides AI systems that analyze data sheets in logs and predict the future, providing accurate safety assessments and alerts.SME aims to go beyond your concerns, not just your expectations!
SME is a leading marine engineering firm located in China that specializes in advanced corrosion protection, aluminum alloy anode, and solutions to prevent marine growth.With decades of industry experience, SME offers comprehensive solutions which include the design, installation and ongoing maintenance of ICCP systems, MGPS, and Plate Heat Exchangers (PHE). Our five thousand square feet and our $10 million inventory are an example of SME's determination and capability to provide high-quality services. Our assistance for hundreds of vessels annually ensures that each project is able to benefit from our knowledge and state of the art technology.SME aims to go beyond the scope of your concerns, not only your expectations!
aluminum alloy anode offers experts Marine Growth Prevention System solutions (MGPS) to guard the cooling system of your vessel from harmful biofouling in the marine environment. Our systems are constructed and designed using precision which guarantees long-lasting performances and minimal maintenance.With decades of expertise in marine engineering SME utilizes its all aspects of MGPS services which include design installation modification maintenance and repair. Our huge inventory will allow us to quickly satisfy any MGPS requirements for spare parts making sure your vessel is protected without interruption.Selecting SME means that you partner with a company that not only offers reliable MGPS solutions but also provides the resources and expertise required to keep your system running functioning at peak efficiency thereby lessening the risk of having to make costly repair costs and downtime because of marine biofouling.