Ships are susceptible to something called corrosion and zinc anodes have them covered! Metals release back into the water, causing corrosion or when metal is worn away and injured by saltwater and other chemicals. This material softens with temperatures high enough to dull metal, and sometimes it will crack. Zinc metal is perfect for stopping this from occurring on ships since it works in the form of a protective fence.
Ships remain in water all the time, and that is hard on steel. This is why we need to protect them in the first place. Zinc anodes sacrifice themselves to corrosion, so the rest of the ship does not have to. Specifically, it acts as a strong shield that shields the ship from being wounded by ocean sea in any way.
Picture this: zinc is essentially a magnet for anything bad that could damage a ship. As the ship sails in saltwater, the zinc anodes take all the salt and other corrosive features to them before it destroys the metal. It does this because it avoids the hull being damaged, which is very key when you are in open waters as weather can change really quick and if you sustain a hole... it turns into a bad day.
What makes zinc anode cool is they are sacrificial. They are thus designed which actually wear and tear or progit from ship rather than the ship metal itself. That is why, every time the ship docks or gets serviced, its old zinc anodes are taken out and new ones are inserted. This protects the boat against corrosion on a regular basis. It would be equivalent to changing the batteries in a toy to keep it functioning!
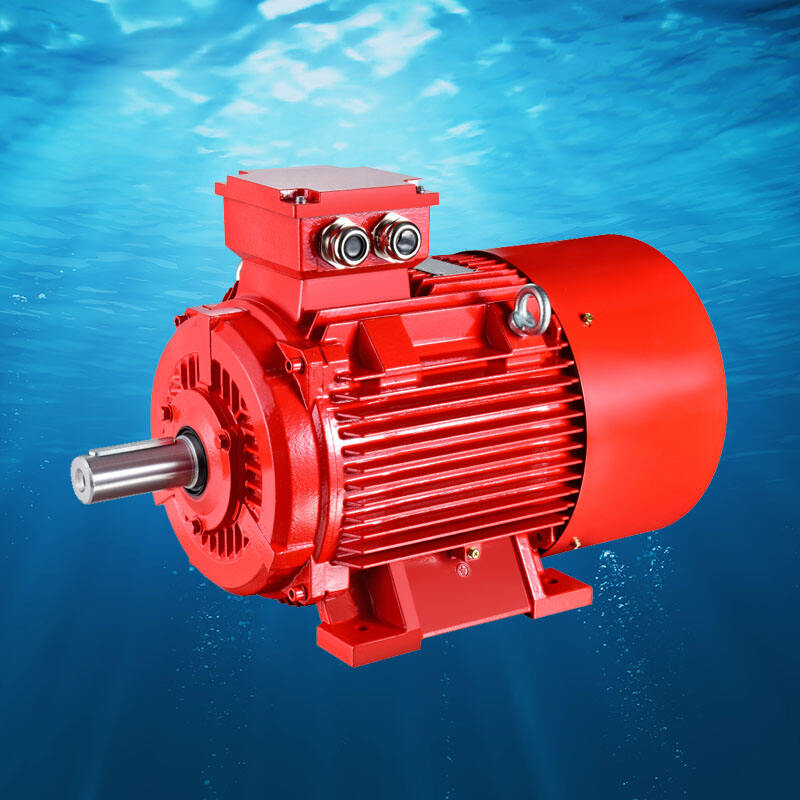
These zinc anodes protect the ship from corrosion, keeping the metal strong and preserving its use. This ensure that the ship is always prepared for whatever comes their way, be it squalor or a sudden obstruction. Over time, the metal of the ship would become weakened since they are constructed using zinc anodes (which could have deteriorated from corrosion), thus can be easily destroyed; a risk for sinking.

That is why zinc anodes are used to avoid corrosion in ships and prevent them from becoming old sooner. They are a special layer that consumes all the evils that would damage the metal of the frame ship! It has helped keep the ship as one of the strongest — and longest-lasting — in any fleet.
This call for a zinc anode is that the zinc anodes work as a shielding layer, They wear out instead of harmful things very easily and can be replaced afford-ably. It indicates, that ships are always safe from corrosion and their metal will remain strong for many years. Keeping your zinc anodes up-to-date is one of the best ways to maintain your ship.
SME's ICCP (Impressed Current Cathodic Protection) systems are zinc anode in ship based on advanced technology and years of expertise in the field. As a leading manufacturer in China We ensure top quality and low maintenance through the accumulation of experience through the handling of hundreds of vessel projects every year.We offer a range of ICCP Solutions, including anodes and spare parts. SME also offers expert modifications or repairs, as well as maintenance which will ensure that your equipment performs optimally throughout its lifetime. We also offer AI systems that analyze data sheets in logs and predict the future, providing accurate safety assessments and alerts.SME strives to be more than your expectations, not only your expectations!
zinc anode in ship offers experts Marine Growth Prevention System solutions (MGPS) to guard the cooling system of your vessel from harmful biofouling in the marine environment. Our systems are constructed and designed using precision which guarantees long-lasting performances and minimal maintenance.With decades of expertise in marine engineering SME utilizes its all aspects of MGPS services which include design installation modification maintenance and repair. Our huge inventory will allow us to quickly satisfy any MGPS requirements for spare parts making sure your vessel is protected without interruption.Selecting SME means that you partner with a company that not only offers reliable MGPS solutions but also provides the resources and expertise required to keep your system running functioning at peak efficiency thereby lessening the risk of having to make costly repair costs and downtime because of marine biofouling.
SME provides an extensive array of Plate Heat Exchanger (PHE) maintenance services tailored to ensure the long-term durability and performance of your equipment. Our services include professional cleaning, thorough inspections, precise pressure testing and sophisticated zinc anode in ship (CIP) processes that permit efficient cleaning on-line, without the need to dismantle the equipment.With a 5,000 square-meter workshop, and a $10 million inventory, we are equipped to manage projects of any scale. The huge capacity, along with our years of expertise and cutting-edge technology for service makes it possible for us to offer a 12-month guarantee on all of our PHE projects.We're committed to quality and accuracy. This helps us minimize risks and increase the lifespan of the heat exchangers you have installed.
SME is a leading marine engineering firm in China with a focus on advanced corrosion protection and heat exchanger services and preventive systems for marine growth.SME has decades of expertise in the industry and offers an array of solutions that encompass installation, design and maintenance of ICCP Systems, MGPS and Plate Heat Exchangers. Our 5,000 square meters and the inventory of over $10 million reflect SME's zinc anode in ship and ability to offer high-quality services. We provide support to hundreds of vessels every year, making sure that each project benefit from our extensive expertise and modern technology.SME is determined to address the scope of your concerns, not only your expectations!